Directive 2010/32/EU: State of The Art in Europe
Six months have passed since D.Lgs. 19/14, the legislative decree to implement Directive 2010/32/EU, which implements the framework agreement negotiated by HOSPEEM (The European Hospital and Healthcare Employers' Association) and EFPSU (European Federation of Public Service Unions) on prevention from sharps injuries in the hospital and healthcare sector.
D.Lgs. 19/14 underlined important topics for prevention from biological risk and highlighted preventive and protective measures:
using and disposing of sharp medical instruments and contaminated waste;
implementing safe working procedures by introducing best practices, using safety-engineered devices, banning of recapping;
using personal protective equipment;
vaccinations;
information and awareness-raising.
To this day, implementing directive 2010/32/EU has resulted into a series of regional guidelines on the use of safety-engineered medical devices, as a preventive measure from accidental sharps injuries. This underlines once more that implementing safety systems does have a great impact on risk reduction.
As is often the case with Italy, implementing the prevention and protection measures outlined in the directive is taking some time. This is why we have decided to look at what is happening in other Member States.
Germany is leading the way, with the guidelines on prevention and safety with biological agents (Biologische Arbeitsstoffe im Gesundheitswesen und in der Wohlfahrtspflege), which contain not only the implementation of the measures outlined in the directive, but also the definition of safe work practices for all tasks and roles.
Another noteworthy example is "Herramienta para la implementación de la Directiva europea de prevención de las lesiones causadas por instrumentos cortantes y punzantes en el sector hospitalario y sanitario", the Spanish guidelines that contain an interesting analysis of the costs/benefits of implementing safety-engineered. This analysis highlights that the costs stemming from accidental sharps injuries far outweigh those of implementing preventive measures.
Moreover, Appendix 3 of the German guidelines includes a risk analysis for each device and kind of hospital procedure:
| FREQUENCY of sharps injuries in healthcare settings | |||||
| Seldom | Sometimes | Often | Frequently | ||
|
RISK by |
Potentially fatal |
Peripheral vein catheters |
Blood drawing needle | ||
| Serious | Intra muscular syringes | Scalpel blades | |||
| Medium | Needles for acupunture | Blood spurt |
Surgical instruments |
||
| Low | No contact with patient | Heparin syringes | Pens for insulin | ||
Table by Prof. Andreas Wittmann, University of Heidelberg, Germany, May 2011.
Preventive measures:
| Using safety devices is vital; vaccination against hepatitis B; mandatory training and awareness-raising. |
| Using safety device is recommended; vaccination against hepatitis B; mandatory training and awareness-raising. |
| Mandatory training of staff to reach the highest possible level of safety. Discontinuing the use of sharps if an alternative is possible. |
* When no safety devices exist, the following measures are recommended: using two pairs of gloves, vaccination against hepatitis B; mandatory training and awareness-raising.
The analysis we have mentioned here points out that using safety devices, vaccination against hepatitis B and training and awareness-raising for the staff are vital measures to manage risk effectively and ensure compliance with directive 2010/32/EU. Where such steps have been taken universally, prevention from biological risk has been effectively carried out.
The other member states are still in the implementation phase of the directive, but it is interesting to see which preventive and protective measures are considered as the most effective in reducing risk from sharps injuries. The following tables sum up this concept: the sample goes from 0 to 100, where 100 represents implementing all required measures, and decreasing values have been assigned on the basis of how often the preventive measure has been implemented.
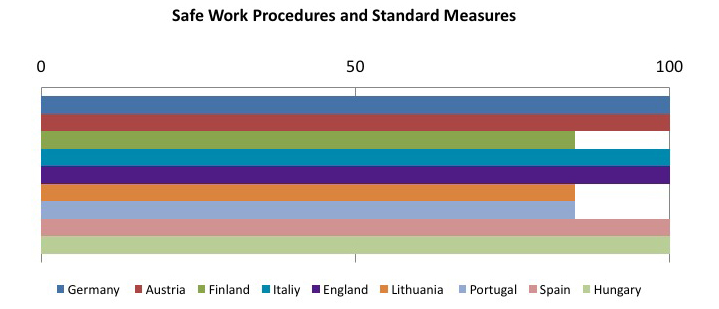
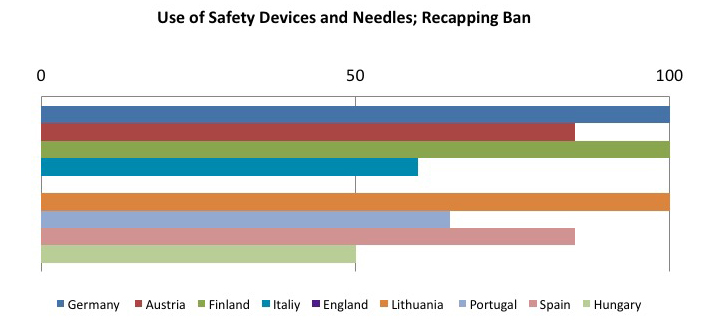
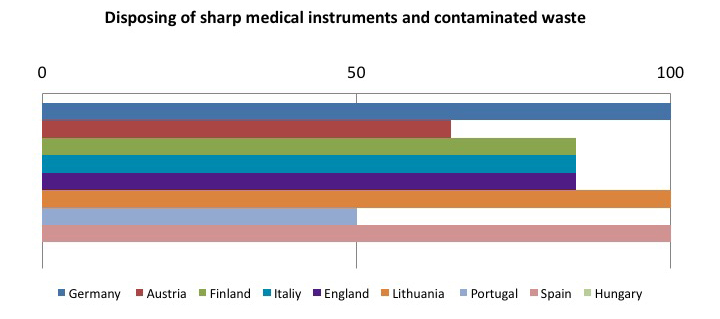
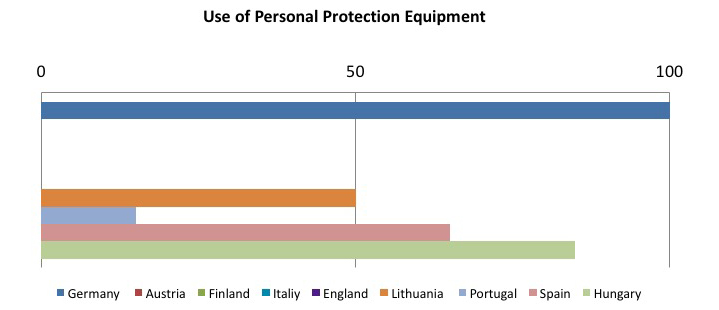
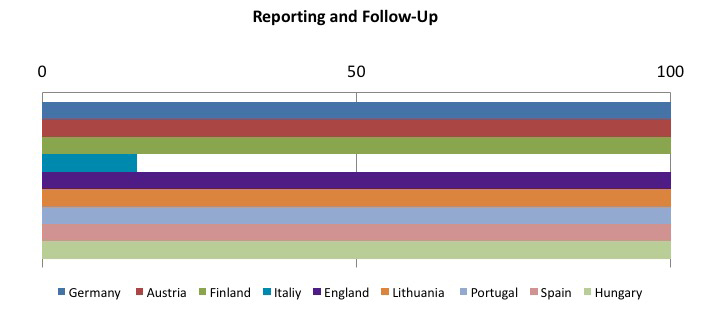
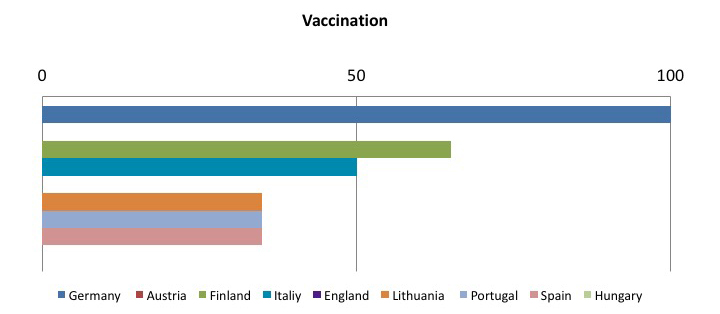

To sum up, the tables show that the most frequently adopted measures in the Member States:
- Safe, effective and shared procedures for needle and contaminated waste disposal:
- Use of safety-engineered sharps;
- Systems underlining the importance of incident reporting
These data compound the need for controlled implementation of safety-engineered medical devices, which appears to be an effective tool in reducing the exposure to risk due to sharps in the hospital and health care setting.




